Method 1
A suspension of 1.36g (0.034 mol) of finely ground sodium hydroxide was prepared in 10 ml of dried DMF. A solution containing 6.0g (0.031 mol) of diphenylacetonitrile in 8 ml of DMF was added thereto at room temperature. After stirring the mixture for 15 minutes, 4.1g (0.034 mol) of 1-dimethylamino-2-chloropropane were added. The reaction mixture was heated with stirring to about 50°C for about 1.5 hours and was then cooled. The cooled reaction mixture was diluted with an equal volume of water and the resulting suspension, containing a mixture of 2,2-diphenyl-4-dimethylaminovaleronitrile and 2,2-diphenyl-3-methyl-4-dimethylamino- butyronitrile formed in the above reaction, was extracted with two 350 ml portions of benzene. The benzene extracts were combined, washed with water and with saturated sodium chloride solution and then dried. Removal of the solvent yielded about 7.83g of the crude reaction product which was shown by vapor phase chromatography to contain 58.4% 2,2-diphenyl-4-dimethylaminovaleronitrile, 29.3% of 2,2-diphenyl-3- methyl-4-dimethylaminobutyronitrile, and 10.8% of starting material diphenylacetonitrile. The methadone intermediate isomers were thus present in a ratio of 66.5:33.5. Recrystallization of the crude reaction product from hexane yielded purified 2,2-diphenyl-4-dimethylaminovaleronitrile.
The above reaction was also carried out employing DMSO in place of DMF as a solvent. The crude product analyzed for 60.6% of the desired valeronitrile isomer, 31.2% of the undesired methyl butyronitrile isomer, and 3.4% of unreacted diphenylacetonitrile (isomer ratio 66:34).
Method 2
A solution of 19.3 g. (0.1 mol.) of diphenylacetonitrile in 60 ml dimethylformamide was added with stirring to a slurry of 8g (0.2 mol) finely ground sodium hydroxide in 40 ml dimethylformamide under nitrogen. The dark red color of the nitrile anion was observed immediately. The mixture was heated to 75°C ±5°C and 14.85g (0.12 mol) 1-dimethylamino-2-chloropropane were added at a rate such that the reaction temperature was maintained in the range 75-80°C with external cooling when necessary. The reaction mixture was stirred at 75°C. under nitrogen for 1 hour, cooled and diluted with 250ml water. The aqueous mixture was extracted with 400 ml. of benzene in three portions. The extracts were combined and the combined extracts were washed with water and with saturated sodium chloride solution, and were then dried over anhydrous sodium sulfate. Removal of the benzene at reduced pressure afforded 26.7g of the crude mixture of isomeric nitriles, shown by VPC analysis to contain 64.8% 2,2-diphenyl-4-dimethylaminovaleronitrile, 34% 2,2-diphenyl-3-methyl- 4-dimethylaminobutyronitrile, and 0.35% unreacted diphenylacetonitrile, the remainder of the material consisting of unidentifed volatile impurities. The reaction was thus 99.6%. The ratio of isomeric nitriles was therefore, 65.6:34.4 in favor of the desired valeronitrile methadone intermediate. The crude product thus obtained was allowed to crystallize from hexane, affording 12.6g (45% of theory based on diphenylacetonitrile) of 2,2-diphenyl-4-dimethylaminovaleronitrile, mp 90-91°C. having a purity of 99%.
Method 3
60g (1.5 moles) of flake sodium hydroxide, 77.2g (0.4 moles) diphenylacetonitrile and 79g (0.5 moles) of 1-dimethylamino-2-choropropane hydrochloride were mixed in an erlenmeyer flask and heated with occasional stirring for 6-7 hours on a steam bath [or an oil bath with the temp at 100°C]. The reaction mixture was then extracted with ether and the ether in turn extracted ith dilute hydrochloric acid [~5% HCl(aq)]. The acid solution was made strongly alkaline with 25% sodium hydroxide solution, and the liberated base extracted with ether. The ether solution was dried over anhydrous potassium carbonate, filtered and the ether distilled off. The residue was vacuum distilled to give 89g of product, boiling at 173-174°C at 1 mmHg. It was then recrystallized from petroleum ether to give 49g (45.7%) of 2,2-Diphenyl-3-methyl-4-dimethylaminobutyronitrile, mp 89-90°C.
Синтез метадона
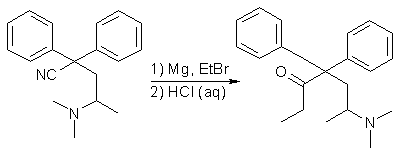
A 500ml distillation apparatus, equipped with a dropping funnel, condenser, stirrer, and drying tubes, was charged with a solution of ethyl magnesium bromide (prepared from 8.21g of magnesium and 35.57g of ethyl bromide) in 130ml of dry ether. A solution of 42g of 2,2-Diphenyl-3-methyl- 4-dimethylaminobutyronitrile in 80ml of hot anhydrous xylene was added over a period of 15 min. Solvent was distilled from the reaction vessel until the temperature of the reaction mixture rose to 70-80°C, and the mixture heated under reflux for an additional 4.25 h. The condenser was then arranged for distillation, and a solution consisting of 65ml of concentrated (37.5%) hydrochloric acid and 65ml of water was added to the hot reaction mixture over a period of 10 min, all of the remaining solvent distilling during this addition. The hot suspension was drawn off and the vessel rinsed with 20ml of 18% HCl. The crude crystalline Methadone hydrochloride, which crystallized upon cooling the combined acid solutions, was collected, dissolved in 240ml of boiling water containing 2g of activated charcoal, the solution heated to boiling, filtered while hot, and the charcoal residue washed with 10ml boiling water. A solution of 6.5g of sodium hydroxide in 10ml water was added to the combined filtrates. The Methadone freebase, which solidified on cooling, was collected, dissolved in 100ml boiling methanol, the solution filtered to remove a small amount of suspended solid, heated to boiling, and diluted with water until it became slightly turbid. After cooling and stirring the solution, the fine white crystals of Methadone base were collected, washed with 4 ml of methanol, and dried in vacuo. The dried Methadone thus obtained weighed 42.86g, melted at 76-78°C (91.9% yield).
Method 2 - Hydrolysis without prior solvent removal
A solution of 50.8g of 2,2-Diphenyl-3-methyl-4-dimethylaminobutyronitrile in 40ml of hot anhydrous xylene (~65°C) was added to a stirred solution of ethyl magnesium bromide (prepared from 8.8 grams of magnesium and 44 grams of ethyl bromide) in 60ml of anhydrous ethyl ether, and the mixture thereafter heated under reflux for 3 h. The condenser was arranged for distillation, and 280 ml of 10% HCl was added to the mixture, and the organic solvent distilled from the reaction mixture by the heat of the ensuing vigorous reaction. The residue was then transferred to a beaker and 100 ml of benzene added, whereupon three layers formed. Upon standing, the Methadone hydrochloride, which crystallized from the oily middle layer, was collected, dried, dissolved in water, the aqueous solution made alkaline with sodium hydroxide, and then cooled. The Methadone separated as a solid and was crystallized from methanol, giving 48.8g (85.7% yield) of Methadone freebase, mp 77-79°C.
Method 3 - Hydrolysis with 2N H2SO4
When the toluene solution of the Grignard complex from ethylmagnesium iodide and 2,2-Diphenyl-3-methyl- 4-dimethylaminobutyronitrile was heated with an added excess of 2N (1M) sulfuric acid on a boiling water-bath for 30 minutes, Methadone sulfate crystallized out on cooling. It was filtered off and suspended in water, and excess 25% sodium hydroxide solution was added to liberate the free base, which was extracted with ether. After drying of the etheral solution over MgSO4, Methadone hydrochloride was precipitated by neutralization with ethanolic hydrogen chloride, and obtained pure in 91% yield (mp 232-233°C).
1-Dimethylamino-2-chloropropane
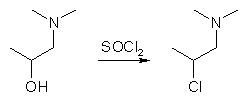
Both 1-dimethylamino-2-propanol (bp 124°C) and 2-dimethylamino-1-propanol (bp 145°C) can be chlorinated by thionyl chloride in chloroform, to form the respective hydrochlorides of the corresponding alkyl chlorides, with mp 185-186°C and mp 104°C. The hydrochloride salt of the latter is soluble in chloroform, so it is not suitable for separation from the chloroform mother liquor by simple filtration, like the hydrochloride of the former. However, if the salts are made into the freebase and distilled, both rearrange into 1-dimethylamino- 2-chloropropane (bp 60-63°C/100mmHg) through the cyclic intermediate aziridinium salt (1,1,2-trimethyl- aziridinium chloride, see the introducory part above for its structure), as does the salts upon melting (mp 191-191.5°C).
2.2g 1-dimethylamino-2-chloropropane hydrochloride was dissolved in an equal amount of water and 1.5ml 20% NaOH was added and thorougly shaken. The freebase 1-dimethylamino-2-chloropropane, being insoluble in the aqueous alkaline solution, separated and was extracted with 2x5ml diethyl ether, and the combined etheral layers were dried over MgSO4, and the ether evaporated to give an oily residue consisting of 0.8g 1-dimethylamino-2-chloropropane.
If purer 1-dimethylamino-2-chloropropane freebase is desired, the hydrochloride salt can be turned into the freebase and distilled, by Schultz' method
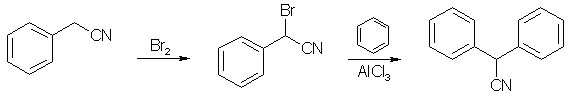
This is an adaption of the Friedel-Crafts method for synthesizing diphenyl- acetonitrile, which minimizes the exposure to the intermediate alpha-bromo-alpha-phenylacetonitrile, which is a powerful
Diphenylacetonitrile from Benzyl Cyanide
In a five-liter, three-necked flask equipped with a dropping funnel whose stem extends below the surface of the liquid, a mercury-sealed stirrer and a reflux condenser protected by a calcium chloride tube is placed 441g (3.76 moles, 290 ml) of
The reaction mixture is cooled to 20°C. Stirring is continued and 507 g. (3.81 moles) of powdered anhydrous aluminum chloride is added in portions in the course of about one hour with the usual precautions (Note 2).
The temperature in this period is maintained at 20-25°C. When the addition of catalyst is complete, the temperature of the mixture is slowly raised. In about fifteen minutes, when the temperature has reached 35-40°C, vigorous evolution of hydrogen bromide commences. Upon abatement of the reaction, the mixture is heated under reflux for 60-90 minutes and then cooled to room temperature.
It is poured slowly and with stirring into a mixture of 1800 g. of ice and 760 ml. of 1:1 hydrochloric acid. The layers are separated. The aqueous portion is extracted twice with 800-ml. portions of benzene. The combined benzene extracts are-washed successively with one liter of water, one liter of 5% sodium carbonate and one liter of water. The washings are discarded; the benzene solution is dried over 250g. of anhydrous sodium sulfate. The benzene is distilled at atmospheric pressure and the residue is distilled under reduced pressure using a steam-heated condenser; bp 160-170°C/5 mmHg. The crude product is recrystallized from methanol (0.5 mL/g); yield (in two crops) 585g (80% based on benzyl cyanide) ; mp 73-74°C.
In a 2-liter; four-necked flask, equipped with a condenser, stirrer, thermometer, and addition funnel, were placed anhydrous benzene (4.5 liters) and technical anhydrous aluminum chloride (7 pounds). Operations were conducted in a well ventilated hood since hydrogen chloride and hydrogen cyanide were evolved. The flask and contents were cooled by means of an ice bath.
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
A suspension of 1.36g (0.034 mol) of finely ground sodium hydroxide was prepared in 10 ml of dried DMF. A solution containing 6.0g (0.031 mol) of diphenylacetonitrile in 8 ml of DMF was added thereto at room temperature. After stirring the mixture for 15 minutes, 4.1g (0.034 mol) of 1-dimethylamino-2-chloropropane were added. The reaction mixture was heated with stirring to about 50°C for about 1.5 hours and was then cooled. The cooled reaction mixture was diluted with an equal volume of water and the resulting suspension, containing a mixture of 2,2-diphenyl-4-dimethylaminovaleronitrile and 2,2-diphenyl-3-methyl-4-dimethylamino- butyronitrile formed in the above reaction, was extracted with two 350 ml portions of benzene. The benzene extracts were combined, washed with water and with saturated sodium chloride solution and then dried. Removal of the solvent yielded about 7.83g of the crude reaction product which was shown by vapor phase chromatography to contain 58.4% 2,2-diphenyl-4-dimethylaminovaleronitrile, 29.3% of 2,2-diphenyl-3- methyl-4-dimethylaminobutyronitrile, and 10.8% of starting material diphenylacetonitrile. The methadone intermediate isomers were thus present in a ratio of 66.5:33.5. Recrystallization of the crude reaction product from hexane yielded purified 2,2-diphenyl-4-dimethylaminovaleronitrile.
The above reaction was also carried out employing DMSO in place of DMF as a solvent. The crude product analyzed for 60.6% of the desired valeronitrile isomer, 31.2% of the undesired methyl butyronitrile isomer, and 3.4% of unreacted diphenylacetonitrile (isomer ratio 66:34).
Method 2
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
A solution of 19.3 g. (0.1 mol.) of diphenylacetonitrile in 60 ml dimethylformamide was added with stirring to a slurry of 8g (0.2 mol) finely ground sodium hydroxide in 40 ml dimethylformamide under nitrogen. The dark red color of the nitrile anion was observed immediately. The mixture was heated to 75°C ±5°C and 14.85g (0.12 mol) 1-dimethylamino-2-chloropropane were added at a rate such that the reaction temperature was maintained in the range 75-80°C with external cooling when necessary. The reaction mixture was stirred at 75°C. under nitrogen for 1 hour, cooled and diluted with 250ml water. The aqueous mixture was extracted with 400 ml. of benzene in three portions. The extracts were combined and the combined extracts were washed with water and with saturated sodium chloride solution, and were then dried over anhydrous sodium sulfate. Removal of the benzene at reduced pressure afforded 26.7g of the crude mixture of isomeric nitriles, shown by VPC analysis to contain 64.8% 2,2-diphenyl-4-dimethylaminovaleronitrile, 34% 2,2-diphenyl-3-methyl- 4-dimethylaminobutyronitrile, and 0.35% unreacted diphenylacetonitrile, the remainder of the material consisting of unidentifed volatile impurities. The reaction was thus 99.6%. The ratio of isomeric nitriles was therefore, 65.6:34.4 in favor of the desired valeronitrile methadone intermediate. The crude product thus obtained was allowed to crystallize from hexane, affording 12.6g (45% of theory based on diphenylacetonitrile) of 2,2-diphenyl-4-dimethylaminovaleronitrile, mp 90-91°C. having a purity of 99%.
Method 3
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
60g (1.5 moles) of flake sodium hydroxide, 77.2g (0.4 moles) diphenylacetonitrile and 79g (0.5 moles) of 1-dimethylamino-2-choropropane hydrochloride were mixed in an erlenmeyer flask and heated with occasional stirring for 6-7 hours on a steam bath [or an oil bath with the temp at 100°C]. The reaction mixture was then extracted with ether and the ether in turn extracted ith dilute hydrochloric acid [~5% HCl(aq)]. The acid solution was made strongly alkaline with 25% sodium hydroxide solution, and the liberated base extracted with ether. The ether solution was dried over anhydrous potassium carbonate, filtered and the ether distilled off. The residue was vacuum distilled to give 89g of product, boiling at 173-174°C at 1 mmHg. It was then recrystallized from petroleum ether to give 49g (45.7%) of 2,2-Diphenyl-3-methyl-4-dimethylaminobutyronitrile, mp 89-90°C.
Повідомлення об'єднано автоматично:
Синтез метадона
Methadone
Method 1 - Hydrolysis with 13 eqv. HCl after solvent removalБудь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
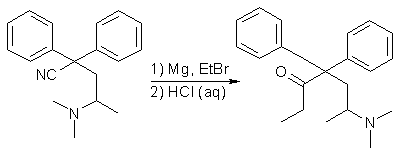
A 500ml distillation apparatus, equipped with a dropping funnel, condenser, stirrer, and drying tubes, was charged with a solution of ethyl magnesium bromide (prepared from 8.21g of magnesium and 35.57g of ethyl bromide) in 130ml of dry ether. A solution of 42g of 2,2-Diphenyl-3-methyl- 4-dimethylaminobutyronitrile in 80ml of hot anhydrous xylene was added over a period of 15 min. Solvent was distilled from the reaction vessel until the temperature of the reaction mixture rose to 70-80°C, and the mixture heated under reflux for an additional 4.25 h. The condenser was then arranged for distillation, and a solution consisting of 65ml of concentrated (37.5%) hydrochloric acid and 65ml of water was added to the hot reaction mixture over a period of 10 min, all of the remaining solvent distilling during this addition. The hot suspension was drawn off and the vessel rinsed with 20ml of 18% HCl. The crude crystalline Methadone hydrochloride, which crystallized upon cooling the combined acid solutions, was collected, dissolved in 240ml of boiling water containing 2g of activated charcoal, the solution heated to boiling, filtered while hot, and the charcoal residue washed with 10ml boiling water. A solution of 6.5g of sodium hydroxide in 10ml water was added to the combined filtrates. The Methadone freebase, which solidified on cooling, was collected, dissolved in 100ml boiling methanol, the solution filtered to remove a small amount of suspended solid, heated to boiling, and diluted with water until it became slightly turbid. After cooling and stirring the solution, the fine white crystals of Methadone base were collected, washed with 4 ml of methanol, and dried in vacuo. The dried Methadone thus obtained weighed 42.86g, melted at 76-78°C (91.9% yield).
Method 2 - Hydrolysis without prior solvent removal
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
A solution of 50.8g of 2,2-Diphenyl-3-methyl-4-dimethylaminobutyronitrile in 40ml of hot anhydrous xylene (~65°C) was added to a stirred solution of ethyl magnesium bromide (prepared from 8.8 grams of magnesium and 44 grams of ethyl bromide) in 60ml of anhydrous ethyl ether, and the mixture thereafter heated under reflux for 3 h. The condenser was arranged for distillation, and 280 ml of 10% HCl was added to the mixture, and the organic solvent distilled from the reaction mixture by the heat of the ensuing vigorous reaction. The residue was then transferred to a beaker and 100 ml of benzene added, whereupon three layers formed. Upon standing, the Methadone hydrochloride, which crystallized from the oily middle layer, was collected, dried, dissolved in water, the aqueous solution made alkaline with sodium hydroxide, and then cooled. The Methadone separated as a solid and was crystallized from methanol, giving 48.8g (85.7% yield) of Methadone freebase, mp 77-79°C.
Method 3 - Hydrolysis with 2N H2SO4
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
When the toluene solution of the Grignard complex from ethylmagnesium iodide and 2,2-Diphenyl-3-methyl- 4-dimethylaminobutyronitrile was heated with an added excess of 2N (1M) sulfuric acid on a boiling water-bath for 30 minutes, Methadone sulfate crystallized out on cooling. It was filtered off and suspended in water, and excess 25% sodium hydroxide solution was added to liberate the free base, which was extracted with ether. After drying of the etheral solution over MgSO4, Methadone hydrochloride was precipitated by neutralization with ethanolic hydrogen chloride, and obtained pure in 91% yield (mp 232-233°C).
Повідомлення об'єднано автоматично:
Precursors
1-Dimethylamino-2-chloropropane
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
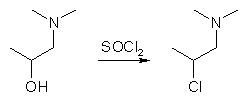
Both 1-dimethylamino-2-propanol (bp 124°C) and 2-dimethylamino-1-propanol (bp 145°C) can be chlorinated by thionyl chloride in chloroform, to form the respective hydrochlorides of the corresponding alkyl chlorides, with mp 185-186°C and mp 104°C. The hydrochloride salt of the latter is soluble in chloroform, so it is not suitable for separation from the chloroform mother liquor by simple filtration, like the hydrochloride of the former. However, if the salts are made into the freebase and distilled, both rearrange into 1-dimethylamino- 2-chloropropane (bp 60-63°C/100mmHg) through the cyclic intermediate aziridinium salt (1,1,2-trimethyl- aziridinium chloride, see the introducory part above for its structure), as does the salts upon melting (mp 191-191.5°C).
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
Experimental
A solution containing 3.77g of 1-dimethylamino-2-propanol and 10ml of chloroform was cooled with stirring to about 0°C. A solution of 5.72g freshly distilled thionyl chloride (SOCl2) in 2ml chloroform was added thereto. The reaction mixture was allowed to come to ambient temperature over 30 minutes, and was then boiled under reflux for another 30 minutes (HCl and SO2 gas is being evolved, use good ventilation). The precipitated material redissolved on heating. 1-dimethylamino-2-chloropropane hydrochloride began to precipitate from the boiling solution. The reaction mixture was cooled, diluted with ether and filtered. The preciptate weighed 5.5g (95% yield). Recrystallization gave pure 1-dimethylamino-2-chloropropane hydrochloride, mp 192-193°C.2.2g 1-dimethylamino-2-chloropropane hydrochloride was dissolved in an equal amount of water and 1.5ml 20% NaOH was added and thorougly shaken. The freebase 1-dimethylamino-2-chloropropane, being insoluble in the aqueous alkaline solution, separated and was extracted with 2x5ml diethyl ether, and the combined etheral layers were dried over MgSO4, and the ether evaporated to give an oily residue consisting of 0.8g 1-dimethylamino-2-chloropropane.
If purer 1-dimethylamino-2-chloropropane freebase is desired, the hydrochloride salt can be turned into the freebase and distilled, by Schultz' method
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
:30g of 1-dimethylamino-2-chloropropane hydrochloride was dissolved in 40-50ml water and made strongly basic with 20% sodium hydroxide solution. The chloroamine layer was separated, dried over solid potassium hydroxide and vacuum distilled under a weak vacuum, bp 62-63°C/100-110mmHg. Yield 19g (82%).
Diphenylacetonitrile

This is an adaption of the Friedel-Crafts method for synthesizing diphenyl- acetonitrile, which minimizes the exposure to the intermediate alpha-bromo-alpha-phenylacetonitrile, which is a powerful
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
, and also gives diphenylacetonitrile in an overall yield of 80% based on reacted
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
.Diphenylacetonitrile from Benzyl Cyanide
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
In a five-liter, three-necked flask equipped with a dropping funnel whose stem extends below the surface of the liquid, a mercury-sealed stirrer and a reflux condenser protected by a calcium chloride tube is placed 441g (3.76 moles, 290 ml) of
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
. Stirring is started and the cyanide is heated to 105-110°C by means of an oil-bath. Now 608g (3.80 moles, 195 ml) of bromine is added in the course of 60-90 minutes. Throughout this period the temperature is maintained within the range indicated above. The hydrogen bromide evolved may be absorbed in a water-trap. After addition is complete, two liters of dry benzene is added and the mixture is heated under reflux for about one hour, until virtually all the hydrogen bromide has escaped. The dropping funnel is now instantly replaced by a solid rubber stopper (Note 1).The reaction mixture is cooled to 20°C. Stirring is continued and 507 g. (3.81 moles) of powdered anhydrous aluminum chloride is added in portions in the course of about one hour with the usual precautions (Note 2).
The temperature in this period is maintained at 20-25°C. When the addition of catalyst is complete, the temperature of the mixture is slowly raised. In about fifteen minutes, when the temperature has reached 35-40°C, vigorous evolution of hydrogen bromide commences. Upon abatement of the reaction, the mixture is heated under reflux for 60-90 minutes and then cooled to room temperature.
It is poured slowly and with stirring into a mixture of 1800 g. of ice and 760 ml. of 1:1 hydrochloric acid. The layers are separated. The aqueous portion is extracted twice with 800-ml. portions of benzene. The combined benzene extracts are-washed successively with one liter of water, one liter of 5% sodium carbonate and one liter of water. The washings are discarded; the benzene solution is dried over 250g. of anhydrous sodium sulfate. The benzene is distilled at atmospheric pressure and the residue is distilled under reduced pressure using a steam-heated condenser; bp 160-170°C/5 mmHg. The crude product is recrystallized from methanol (0.5 mL/g); yield (in two crops) 585g (80% based on benzyl cyanide) ; mp 73-74°C.
Notes
- The equipment may be originally assembled so that one of the side-necks of the flask carries a two-necked adapter. Then no detachment need be made, and all possibility of exposure to alpha-bromo-alpha-phenylacetonitrile can be eliminated.
- It is convenient to weigh the aluminum chloride into an Erlenmeyer flask and to attach the latter by a rubber sleeve to the available neck of the flask.
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
In a 2-liter; four-necked flask, equipped with a condenser, stirrer, thermometer, and addition funnel, were placed anhydrous benzene (4.5 liters) and technical anhydrous aluminum chloride (7 pounds). Operations were conducted in a well ventilated hood since hydrogen chloride and hydrogen cyanide were evolved. The flask and contents were cooled by means of an ice bath.
Будь ласка,
Увійти
або
Реєстрація
щоб переглянути вміст URL-адреси!
(1330 g) was added gradually during a period of 31/2 hours to the stirred reaction mixture, which was maintained at a temperature of 10-20°C. After the addition of the mandelonitrile, the reaction, mixture was heated slowly to a temperature of 75°C and maintained at this temperature, for one hour. After cooling the reaction mixture, water was added cautiously through the addition funnel. Cooling was continued to control the exothermic reaction while a total of 3 liters of water was added. Then excess benzene was removed by steam distillation. The product was present as an oil which solidified on cooling and was filtered from the aqueous part and washed with water. The crude product (1680g, 87% yield) was dissolved in hot methanol (5 L.) and decolorized with activated carbon. The alcoholic solution was chilled and the product which crystallized was filtered. A second recrystallization from methanol (1.6 L) yielded 1464 g. (76% yield) of dry purified diphenylacetonitrile, melting point 73-75°C.References
- E. Schultz, Reaction of Aminoalkylhalides and Diphenylacetonitrile, JACS 69, 188 (1947)
- W. Brode, Rearrangement of the 1,2-dimethylaminochloropropanes, JACS 69, 724 (1947)
- E. Schultz, The Structure of Amidone, JACS 69, 2454-2459 (1947)
- N. Easton, Synthesis and Confirmation of the Amidone Structure, JACS 69, 2941-2942 (1947)
- C. Barnett, Modification of Methadone Synthesis Process Step,
Будь ласка, Увійти або Реєстрація щоб переглянути вміст URL-адреси!
- E. Schulz, Rearrangements of 1,2-dimethylaminochloropropanes, JACS 70, 48 (1948)
- D. Ginsburg, Diphenylacetonitrile, JACS 71, 2254 (1949)
- M. Bockmuhl, Über eine neue Klasse von analgetisch wirkenden Verbindungen
Будь ласка, Увійти або Реєстрація щоб переглянути вміст URL-адреси!
- W. B. Reid, Process for Preparing 4,4-Diphenyl-6-dimethylamino-heptanone-3,
Будь ласка, Увійти або Реєстрація щоб переглянути вміст URL-адреси!
- Casy & Parfitt, Opioid Analgesics - Chemistry and Receptors, p 303-332, Plenum Press (1986)
- A. A. Larsen, JACS 70, 4 194 (1948)
- J. W. Cusic, An Improvement on the Process for Making Amidone, JACS 71, 3546 (1949)
- L. C. Cheney, Ketimines and Acylketimines Related to Amidone, JACS 71, 53 (1949)
- A. L. Morrison, Synthesis of Compounds Related to Amidone, JCS 1478 (1950)
- Clarke, JACS 55, 4571 (1933)
- A. H. Homeyer and J. S. Splitter, Preparation of certain diarylacetonitriles,
Будь ласка, Увійти або Реєстрація щоб переглянути вміст URL-адреси!